RELISTOR injection provided reliable and rapid relief1,10
In a clinical study of adult CNCP patients with OIC


PERCENTAGE OF PATIENTS
STUDY DESIGN: Study 2 was a 4-week, multicenter, double-blind, randomized, placebo-controlled, phase 3 study. The efficacy of RELISTOR injection was evaluated in 312 patients with CNCP for which they were taking opioids. All patients had OIC, defined as <3 SBMs per week and at least one additional symptom of constipation.1
†A responder was defined as a patient with 3 or more SBMs per week for each of the 4 weeks in the double-blind period.1
- RELISTOR injection increased the number of weekly SBMs vs placebo1,10
- SBM‡ achieved within 4 hours of first dose1,10
- – 33% of patients taking RELISTOR 12-mg injection (n=150)1,10
- – 10% placebo (n=162)1,10
- – Significant difference (P<.001)10
‡SBM is defined as bowel movement without the use of any laxative in previous 24 hours.1
Significantly more patients taking RELISTOR injection experienced an SBM§ within 4 hours of the first dose1,12,13
In 2 clinical studies of OIC in adult patients with advanced illness, including many patients with active cancer


PERCENTAGE OF PATIENTS
STUDY DESIGNS: IN ADULT PATIENTS WITH ADVANCED ILLNESS
STUDY 4 was a multicenter, double-blind, randomized, placebo-controlled study; 154 patients with advanced illness and OIC received a single subcutaneous dose of RELISTOR injection or placebo.1,12
STUDY 5 was a 2-week, multicenter, double-blind, randomized, placebo-controlled trial followed by a subsequent 3-month, open-label extension study. The efficacy of RELISTOR was evaluated in 133 patients.1,13
- In adult patients with advanced illness, RELISTOR injection delivered rapid SBMs1,12,13,§
- In Study 5, significantly more patients in the RELISTOR injection cohort (52%; n=62) experienced laxation within the first 4 hours after at least 2 of the first 4 doses vs 9% for placebo (n=71; P<.0001)1,13
Approximately 50% of patients who had an SBM within 4 hours of first dose in Studies 4 and 5 experienced an SBM§ within 30 minutes12,13
§SBM is defined as laxation without the use of a rescue laxative.12,13
§SBM is defined as laxation without the use of a rescue laxative.12,13
RELISTOR injection has a well-established safety profile in patients with advanced illness and CNCP1
Adverse reactions in a 4-week, double-blind, placebo-controlled period of clinical study of RELISTOR injection in adult patients with OIC and CNCP (Study 2)1
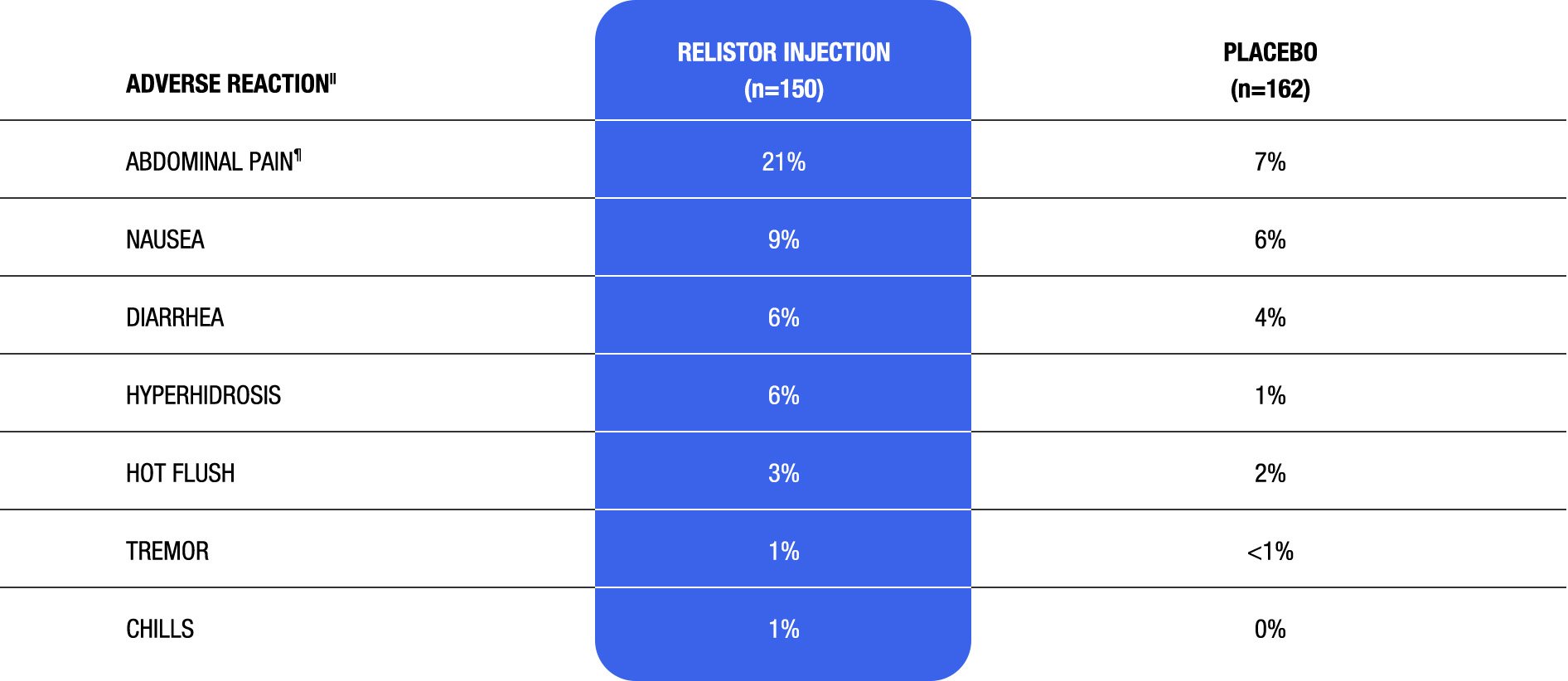

||Adverse reactions occurring in at least 1% of patients receiving RELISTOR injection 12 mg subcutaneously once daily and at an incidence greater than placebo.1
¶Includes: abdominal pain, upper abdominal pain, lower abdominal pain, abdominal discomfort, and abdominal tenderness.1
- The safety of RELISTOR injection was also evaluated in Study 3, a 48-week, open-label, uncontrolled trial in 1034 adult patients with OIC and CNCP1
- – The adverse reactions seen in this study were similar to those observed during the 4-week, double-blind period of Study 2
Adverse reactions from all doses in double-blind, placebo-controlled clinical studies of RELISTOR injection in adult patients with OIC and advanced illness (Studies 4 and 5)1

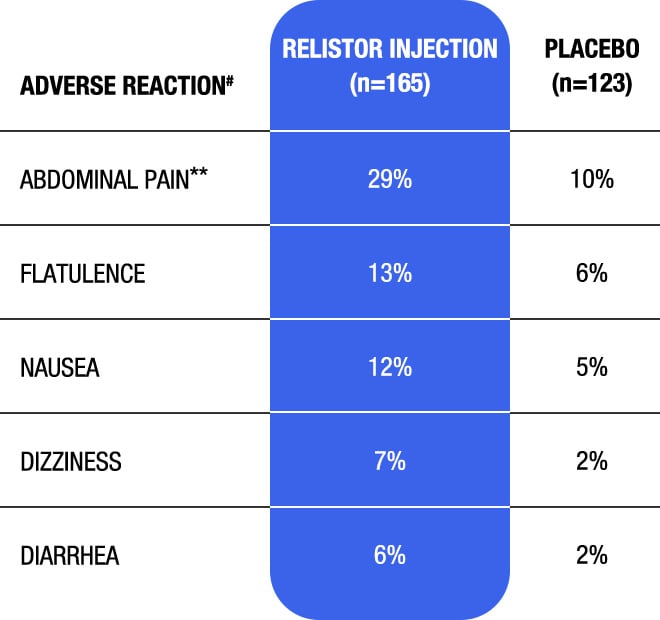
#Adverse reactions occurring in at least 5% of patients receiving all doses of RELISTOR injection (0.075, 0.15, and 0.3 mg/kg) and at an incidence greater than placebo.1
**Includes: abdominal pain, upper abdominal pain, lower abdominal pain, abdominal discomfort, and abdominal tenderness.
- Adverse reactions of abdominal pain, diarrhea, hyperhidrosis, anxiety, rhinorrhea, and chills may reflect symptoms of opioid withdrawal1








