EMERGENCY
MEDICINE
When working in the
emergency department (ED)
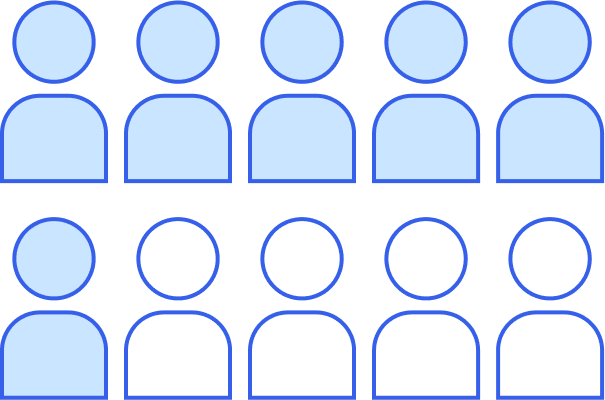
The ED often sees patients with OIC. OIC occurs in 40% to 80% of all patients receiving an opioid medication for chronic pain.3-5
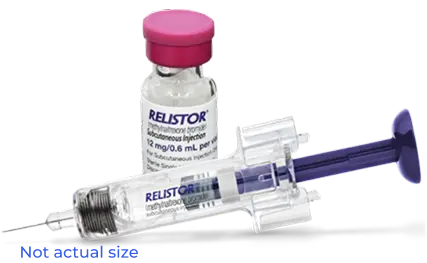
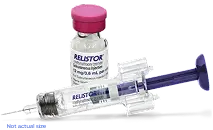
RELISTOR injection is available in vials and pre-filled syringes1
REFERENCES: 1. RELISTOR [prescribing information]. Bridgewater, NJ: Salix Pharmaceuticals. 2. Glare P, Walsh D, Sheehan D. The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care. 2006;23(3):229-235. 3. Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European patient survey (PROBE 1). Pain Med. 2009;10(1):35-42. 4. Kalso E, Edwards JE, Moore AR, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372-380. 5. Hjalte F, Berggren AC, Bergendahl H, Hjortsberg C. The direct and indirect costs of opioid-induced constipation. J Pain Symptom Manage. 2010;40(5):696-703.